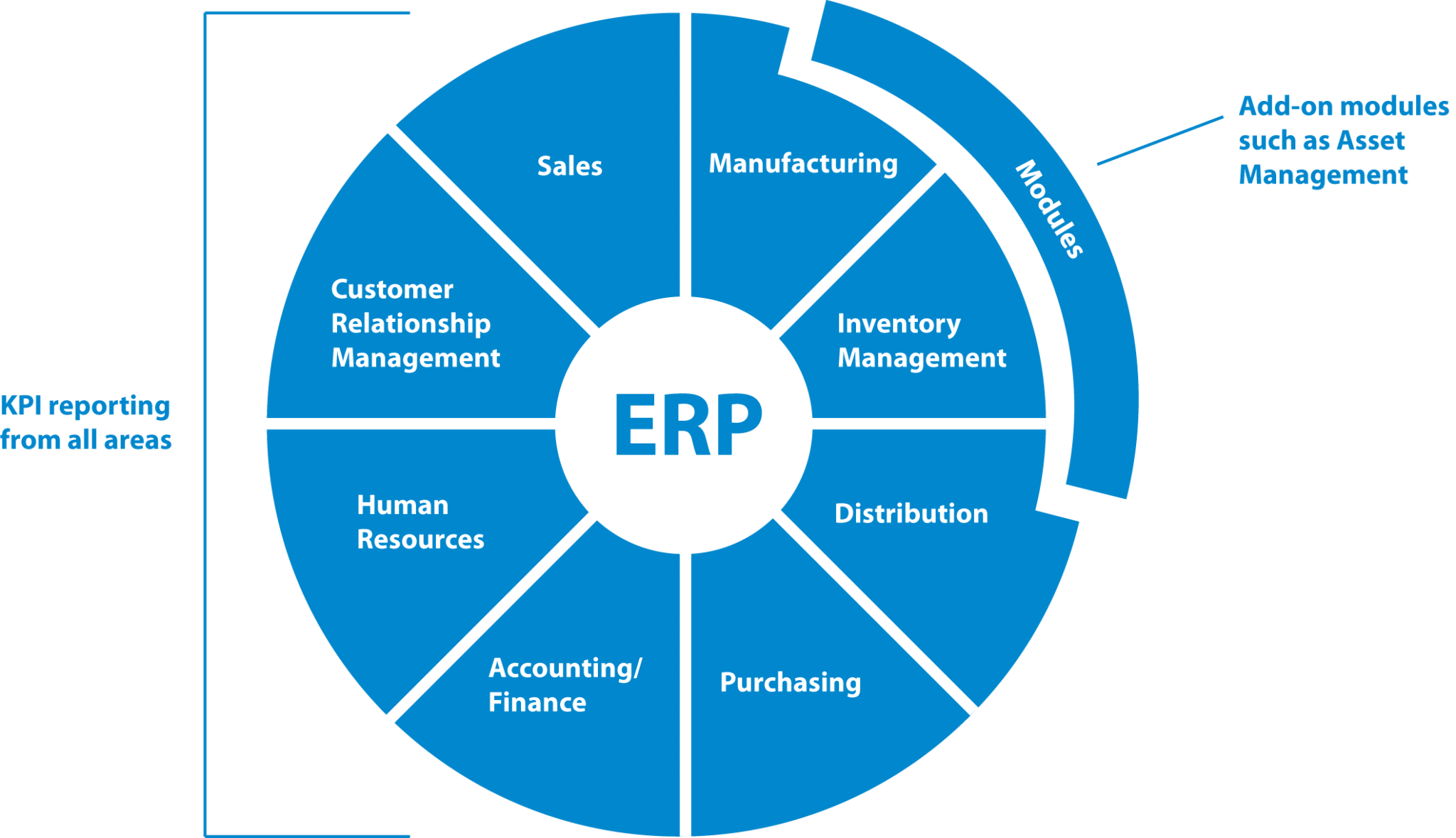
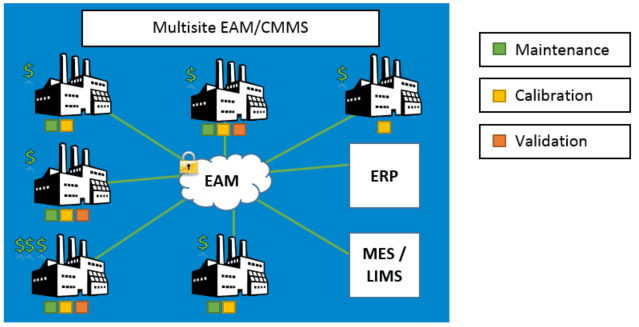
Why Regulatory Asset Management Should be Your Initial GMP System Investment
The Essential Foundational Investment for any GMP Program is an EAM System. Before implementing other quality systems like a Quality Management System (QMS) or a Manufacturing Execution System (MES), your […]