In the previous blog in this series, I discussed the FDA’s new Office of Pharmaceutical Quality and its overarching goals. There is no better summary of what the FDA hopes to achieve and monitor with the OPQ, than a quote taken from an FDA PowerPoint: “a maximally efficient, agile, flexible pharmaceutical manufacturing sector that reliably produces high quality drug products without extensive regulatory oversight.”
What does the Office of Pharmaceutical Quality mean for the Life Sciences Industry?
With the Office’s focus specifically on Pharmaceutical manufacturers, there is not an immediate pressure for companies in other segments of the industry including Medical Device and Biotech to change. However, OPQ represents the FDA’s overarching desire for all companies to adapt their business processes to focus on quality rather than compliance.
In a recent PowerPoint presentation given by the FDA, they highlighted 3 goals for OPQ:
- “Achieve product quality without extensive regulatory oversight” – The burden to achieve product quality needs to fall to manufacturers.
- “Reduce product-related shortages and quality related recalls” – Occurs through the improvement of product quality.
- “Induce the right behavior and responsibility for the industry”.
These same goals can be applied across all Life Sciences companies – beyond the Pharmaceutical Segment.
The FDA is looking to work in partnership with companies to achieve these goals by improving inspection techniques, collecting and monitoring data from facilities, and developing standards for quality – through consistent metrics. Again, while the FDA’s immediate focus is on pharmaceuticals, there is significant value for other regulated industry segments to adopt leading quality programs.
Key Metrics for Quality Manufacturing
With over 25 years in the Life Sciences industry, we’ve witnessed hundreds of customers implement our software Blue Mountain RAM as their EAM/CMMS systems. With each of these implementations, we’ve gained a unique perspective on how the industry leverages EAM/CMMS applications to monitor compliance risks and overall quality within their facilities.
By managing maintenance, calibration, and validation in a single, consolidated system, companies are able to better manage compliance risks. When all data is collected into an EAM/CMMS application, reporting can help to identify key trends across entire facilities and on an individual equipment basis. While these trends help to improve quality and manage compliance risks, they will also aid in balancing investment, so that companies are improving product quality, while ensuring adequate return on investment.
Work & Asset Analysis
By looking at an overview of all assets in a facility, managers gain an enormous perspective and insight into managing compliance risks and balancing investment. With an EAM/CMMS, management can easily monitor all work being done in a facility for overarching trends. If any of these trends are outside the norm, managers can drill down into reports to view the equipment causing any outlying results. This ability to drill down is essential to adequately review performance, foresee potential failures and therefore better manage compliance risks. Here are some examples of typical equipment analysis metrics, trending reports and KPIs:
- Mean Time Between Failure (MTBF)
- Planned vs. Unplanned Work
- Overall Bad Actor Rating – which weighs unplanned work, failed calibrations, usage, and cost overages
- Past Due Work
- Calibration Interval Analysis
- Lifecycle Cost Analysis
- Average Time ‘In Progress’ for Maintenance, Calibration and Validation Work
- Time to Complete Work Orders
- Trending Asset Failures
- Asset Maintenance and Calibration Cost Trending

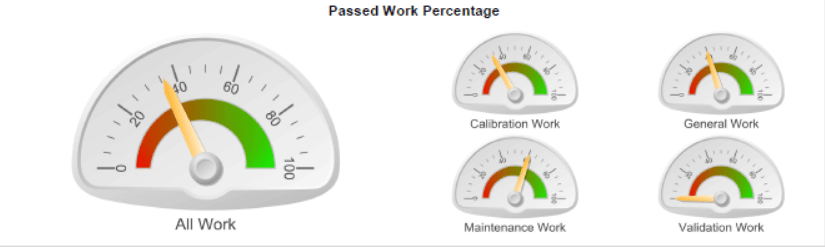
This is an example of a work analysis report showing the percentage of passed work.
All of these metrics provide the necessary information to make strategic decisions for high-risk assets given the assets’ maintenance and calibration history. From a facility’s perspective, managers can analyze work done on a particular manufacturing line and immediately detect any compliance risks by looking for failing equipment or equipment with increasing costs.
Not only does an EAM/CMMS application prove a necessity in managing compliance risks, but also an avenue to balance investment and equipment performance.
For more information on Managing Compliance Risks with EAM/CMMS, view our recorded webinar.
Check out the other blogs in our Managing Compliance Risks with EAM/CMMS series:
- Managing Compliance Risks with EAM
- Focus on Quality Not Compliance: FDA’s New Office of Pharmaceutical Quality
- Monitor Quality with Metrics from EAM / CMMS
- EAM/CMMS Purpose Built for Life Sciences
- Core EAM/CMMS Functionality for Compliance
- Leveraging EAM Functionality to Manage Compliance Risks
Sources: